Background: Myelodysplastic Syndromes (MDS) are a group of myeloid malignancies affecting the hematopoietic stem and progenitor cells and occurring mainly in elderly individuals. Two hypomethylating agents (HMA), Azacitidine (AZA) and Decitabine (DAC), have shown efficacy in treatment of MDS patients and received approval based on randomized controlled trials (RCTs) more than a decade ago. Since then, many publications have reported real world experience on HMA treatment in MDS patients, which showed a reduced efficacy of HMA than the RCT. The reasons for this difference have been extensively discussed but a systematic review never performed.
Aim: We performed a systematic literature review and meta-analysis to compare the efficacy of real world data with randomized controlled trials and to identify potential cofactors for different outcomes.
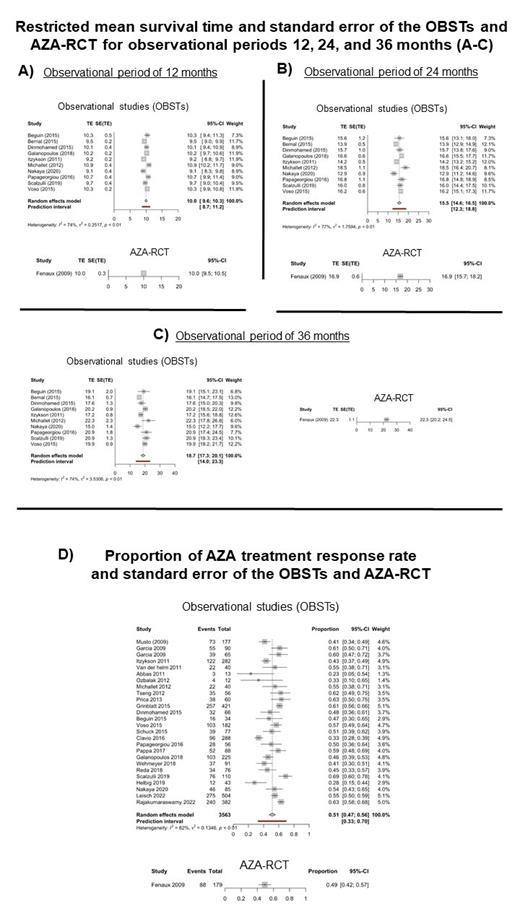
Material and Methods: We performed a systematic literature search from observational studies (OBSTs) describing real world experience of AZA treatment in MDS patients using Embase, Ovid Medline, and Web of Science databases. Patient characteristics, IPSS/IPSS-R, and outcome (response rates, overall survival) were collected from OBSTs that were performed between 2003 and 2023. Kaplan-Meier curves with log-rank tests were used to assess differences in survival outcomes. An inverse variance weighted meta-analysis was performed by using restricted mean survival times or proportions (response rate outcome). The restricted mean survival time and the standard error at 12, 24 and 36 months was calculated by using the R package survival. The pooled effect and prediction intervals from random effect models were reported.
Results: In our systematic literature search, data from OBSTs describing real world experience of AZA treatment in MDS patients could be extracted and compared to the only RCT published with AZA (Fenaux P et al., Lancet Oncology 2009). Patient and disease characteristics were similar for AZA-RCT and OBST (respect of gender ratio, median age, IPPS/IPSS-R). Restricted mean survival time and standard error of the OBSTs and AZA-RCT showed no significant difference for the time points at 12 months (10.0 [95%-CI: 9.6; 10.3] months (OBSTs) vs 10.0 [95%-CI: 9.5; 10.5] months (AZA-RCT); figure part A) and 24 months (15.5 [95%-CI: 14.6; 16.5] months (OBSTs) vs. 16.9 [95%-CI: 15.7; 18.2] months (AZA-RCT); figure part B). At 36 months, the restricted mean survival time of OBSTs was 18.7 [95%-CI: 17.3; 20.1] months compared to 22.3 [95%-CI: 20.2; 24.5] months of the AZA-RCT (figure part C), which was different in favor of the RCT. Using random effect models, the AZA treatment response rate was 0.51 [95%-CI: 0.47; 0.56] for the OBSTs and 0.49 [95%-CI: 0.42; 0.57] for the AZA-RCT (figure part D).
Conclusion: Compared to AZA-RCT data, restricted mean survival time (for time points 12 months and 24 months) and response rate of OBSTs describing real world data of AZA treatment in MDS patients are comparable. Regarding the observation time point at 36 months, the lower restricted mean survival time of the OBSTs compared to AZA-RCT may be most likely explained by study patient selection bias.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal